The Prescription Drug User Fee Program
My second August article looks at the FDA User Fee Program for Prescription Drugs
Update: No live chat on Substack this week - I’m in Minnesota for the Bullpen Mayo Clinic Pitch Fest!
Last week we reviewed the Medical Device User Fee Program, its benefits, and some criticisms. It turns out the FDA has multiple User Fee Programs under different amendments to the FD&C Act. [1] This week, we’ll review the history and mandates for the Prescription Drug User Fee Act (PDUFA) and look at how the program works. Spoiler alert: there is also a PDUFA Small Business program! We’ll finish by looking at the economics of drug discovery and development, something not many people are aware of. So, let’s get started!
What is PDUFA?
The Prescription Drug User Fee Act, or PDUFA, is the first of the FDA’s Congressionally-authorized User Fee programs. Created in 1992, it authorized the FDA to collect fees from companies submitting market applications for certain human drugs and biological products. In addition to funding additional review staff, the fees support programs that support patient science and engagement activities, [2, 3] and certain inspections and clinical research monitoring activities. Since the passage of PDUFA, user fees have played an important role in expediting the drug review and approval process. [4]
PDUFA levies a user fee on certain human prescription drug applications, including FD&C Act section 505(b) applications for new drug approvals and Public Health Service Act section 351(a) licensure of certain biological products. There are several items excluded from the term human drug application, including supplements to such applications and applications with respect to blood components. [4] The amendments must be reauthorized every five years. These renewals were in 1997 (PDUFA II), 2002 (PDUFA III), 2007 (PDUFA IV), 2012 (PDUFA V), 2017 (PDUFA VI), and 2022 (PDUFA VII). This latest reauthorization extends the Act through FY2027. You can find full details on the PDUFA VI reauthorization on this FDA website. [5]
What is PDUFA’s Impact?
Since PDUFA implementation, the FDA has used the program funds to reduce the time needed to evaluate new drugs without compromising standards for safety and efficacy. As we saw in my previous article on MDUFA, more predictable review timelines enable businesses to better plan for standard post-approval manufacturing and marketing activities. They also enable the American people to gain quicker access to new medicines. Later amendments established new performance goals, improved post-market surveillance and risk management, creating new programs to help expedite drug development, and expanding the electronic submissions system, among other improvements. [6] Public and legislative program oversight is facilitated through annual performance and financial reports. [6, 7]
PDUFA VII added new goals and procedures to the program, including:
Performance goals and requirements for the human drug review program,
Planning and transparency requirements for user fee resource management,
Goals and assessment requirements for review staff hiring and retention, and
Information technology and bioinformatics goals. [8]
This last item is the most interesting point. It includes goals to
Enhance transparency and leverage modern technology through engaging industry before implementing changes to electronic submissions platforms and tools and maintaining current data standards;
Developing a data and technology modernization strategy with action plans;
Promoting convergence of data interoperability and interpretability with stakeholders and international consortia such as ICH and ICMRA;
Accelerating modernization at the Center for Biologics Evaluation and Review (CBER);
Monitoring and modernizing the Electronic Submissions Gateway (ESG); and
Leveraging Cloud Technology to progress regulatory digital transformation. [9]
Work on this last point will be facilitated by a 2023 report [10] which found the FDA has taken significant steps to migrate core business functions to multiple cloud environments. However, the agency still faces technical, financial, process, and policy challenges that affect its ability to benefit from these solutions. The FDA Office of Digital Transformation issued a response noting the importance of maintaining federal IT policy, mandates, and safeguards and the very active cyber-crime and economic espionage threats the agency faces. They note the report does not make clear the Agency’s significant “increase in cloud adoption since 2021, moving from 71 to 115 systems and applications hosted in federally authorized cloud environments.” [11] I anticipate this will be the area to watch for the remainder of PDUFA VII’s authorization.
How Does PDUFA Help Therapeutics Startups?
I know what my founders out there are thinking, “Is there a PDUFA Small Business program?” The answer is yes, and your first point of reference should be the guidance document. [12] User fee waivers are available in cases where it’s necessary to protect public health, where the fee represents a barrier to innovation, or in cases where an applicant has limited financial resources. The FDA considers $20M as the marker of limited financial resources, a fact unsurprising when you consider the budgets needed to develop a new drug product (see the next section). There are also exceptions for drugs for rare diseases and submissions by state or federal government entities.
Small business fee waivers are available for companies which:
Employ fewer than 500 employees,
Not have an approved human drug introduced into interstate commerce, and
Are submitting their first human drug application.
The submission timelines are also different than for the MDUFA Small Business program. Drug development companies submit a fee waiver application within a calendar year of their planned NDA or BLA because the waiver has a 1-year expiry date. If they miss the deadline, they can reapply for a new waiver with another 1-year term. Once a company has used a small business fee waiver, they are never again eligible for this part of the PDUFA program. So, while you have an annual deadline to request a fee waiver, it’s not in a fixed time frame like the MDUFA Small Business program.
The Economics of Drug Discovery & Development
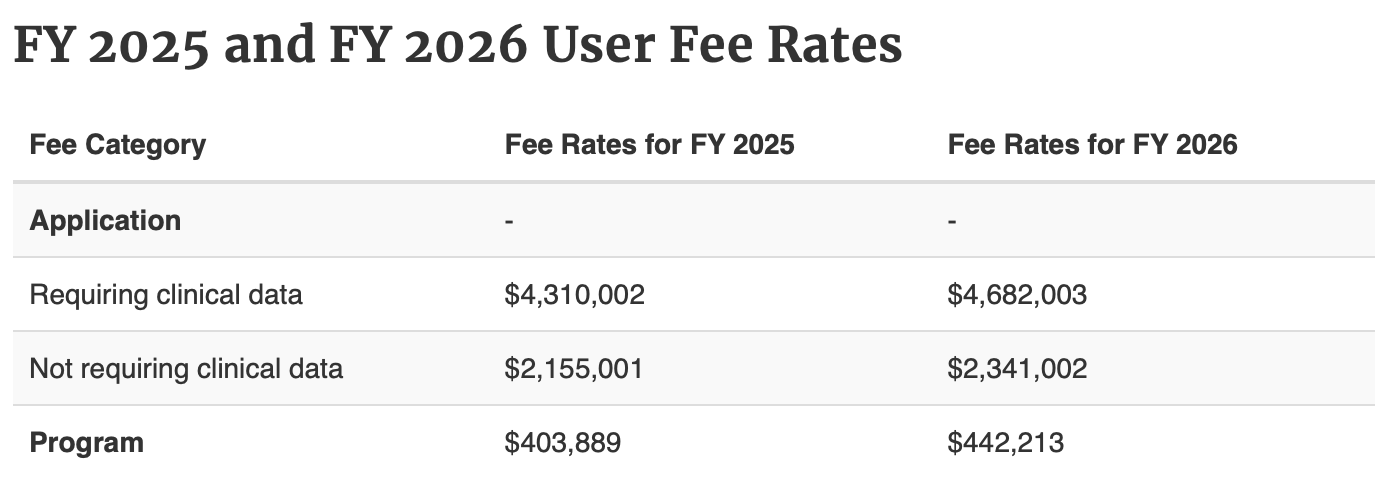
There are two types of prescription drug user fees, one for the application and one for the program. (Figure 1, taken from [4]).
Figure 1. Prescription Drug User Fees FY2025/2026 (from [4]).
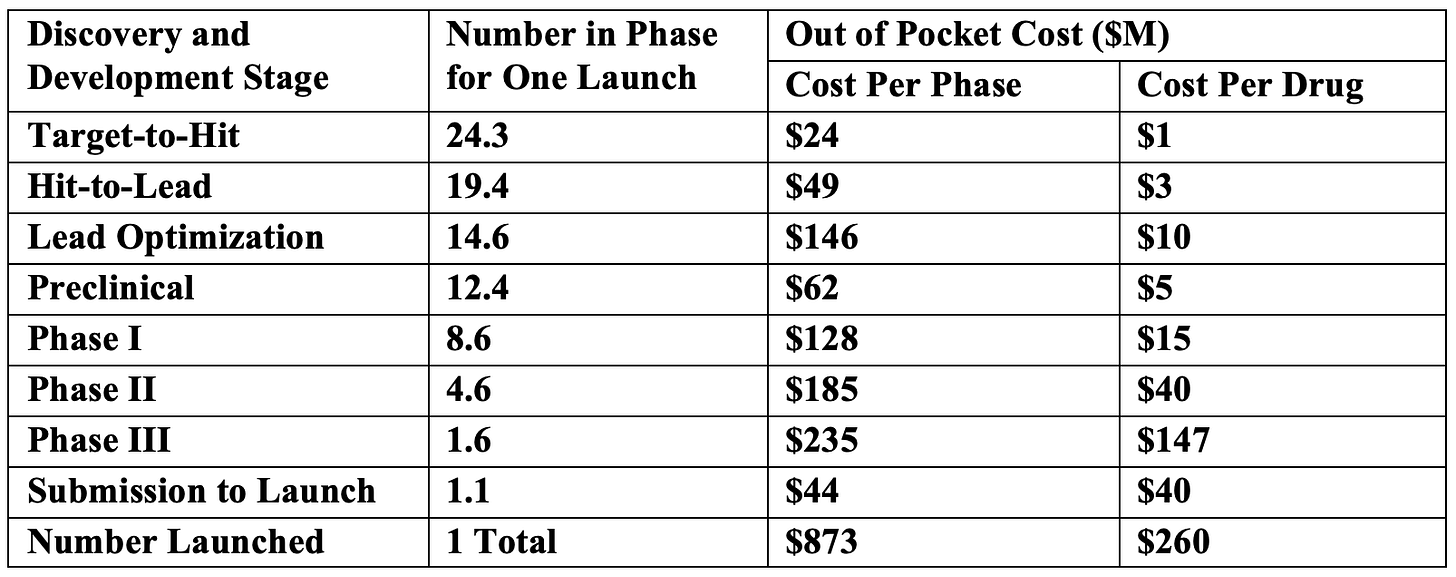
You may find the scale of the FY2025 and FY2026 User Fees shockingly large. However, in comparison to the costs for drug discovery and development (Table 1, adapted from [13]), the user fees for one drug product application are about x% of the total estimated out of pocket costs to develop it.
Table 1. Drug Discovery and Development Costs (adapted from [13]).
These costs don’t include the basic health research which is a necessary first step for identifying a drug target. That research is typically done by universities and colleges, with major funding coming from the NIH. Therefore, the changes being made by U.S. Congress and Administration to deeply cut basic health research will inevitably lead to fewer new medicines in the decades to come. If you want to have new treatments for major medical conditions like cancer, diabetes, & Alzheimer’s, I suggest you call your U.S. Representative and tell them you want basic research funding restored.
The Take Home
User Fee programs have helped the FDA expand its review capabilities and modernize its systems. PDUFA was so successful that it led to multiple additional User Fee programs. [14] These programs are regularly updated through the reauthorization process and receive annual scrutiny via their mandated reports. I’ve published previous articles with performance data that shows these programs improve the predicatability of application review timelines. I’ve also experienced the benefits of the improved digital engagement tools. Every company engaged in drug, device, diagnostic, or digital health innovation should be aware of these programs and use them to their full benefit.
Thanks for reading Thinking Kat! You can help me by liking this post, becoming a paid subscriber, or sharing this publication with your friends.
References
[1] USFDA website, “FDA: User Fees Explained.” Accessed August 8, 2025. https://www.fda.gov/industry/fda-user-fee-programs/fda-user-fees-explained
[2] USFDA website, “CDER Patient-Focused Drug Development.” Accessed August 8, 2025. https://www.fda.gov/drugs/development-approval-process-drugs/cder-patient-focused-drug-development
[3] USFDA website, “Center for Biologics Evaluation and Research Patient Engagement Program.” Accessed August 8, 2025. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/center-biologics-evaluation-and-research-patient-engagement-program
[4] USFDA website, “Prescription Drug User Fee Amendments.” Accessed August 8, 2025. https://www.fda.gov/industry/fda-user-fee-programs/prescription-drug-user-fee-amendments
[5] USFDA website, “PDUFA VII: Fiscal Years 2023 – 2027.” Accessed August 8, 2025. https://www.fda.gov/industry/prescription-drug-user-fee-amendments/pdufa-vii-fiscal-years-2023-2027
[6] USFDA website, “PDUFA Performance Reports.” Accessed August 8, 2025. https://www.fda.gov/about-fda/user-fee-performance-reports/pdufa-performance-reports
[7] USFDA website, “PDUFA Financial Reports.” Accessed August 8, 2025. https://www.fda.gov/about-fda/user-fee-financial-reports/pdufa-financial-reports
[8] USFDA, “PDUFA REAUTHORIZATION PERFORMANCE GOALS AND PROCEDURES FISCAL YEARS 2023 THROUGH 2027.” Accessed August 8, 2025. https://www.fda.gov/media/151712/download?attachment
[9] USFDA website, “PDUFA VII Information Technology and Bioinformatics Goals and Progress.” Accessed August 8, 2025. https://www.fda.gov/industry/prescription-drug-user-fee-amendments/pdufa-vii-information-technology-and-bioinformatics-goals-and-progress
[10] Eagle Hill Consulting, “PDUFA VI Cloud Assessment Summary.” Arlington, VA; December 31, 2023. Accessed August 8, 2025. https://www.fda.gov/media/175118/download?attachment
[11] USFDA Office of Digital Transformation, “FDA Management Response: PDUFA VII Cloud Assessment Survey.” December 31, 2023. Accessed August 8, 2025. https://www.fda.gov/media/175117/download?attachment
[12] USFDA website, “Prescription Drug User Fee Act Waivers, Reductions, and Refunds for Drug and Biological Products Guidance for Industry.” Accessed August 10, 2025. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/prescription-drug-user-fee-act-waivers-reductions-and-refunds-drug-and-biological-products-guidance
[13] Lynn C. Klotz, Ph.D., “What is the Real Drug Development Cost for Very Small Biotech Companies?” In PAREXEL Biopharmaceutical R&D Statistical Sourcebook 2018/2019, Ed. Mark P. Mathieu, PAREXEL International Corporation (Waltham, MA: 2018), p. 292.
[14] USFDA website, “FDA User Fee Programs.” Accessed August 8, 2025. https://www.fda.gov/industry/fda-user-fee-programs
Working with life science startup companies to solve problems, strengthen systems, and build capacity is what I do. If one of your companies or clients is struggling, reach out to me to discuss how I can help your team refocus and emerge triumphant.